Jonah Helmer, Dr. Michael B. Timmons, Dr. Neil Mattson, Cornell University
Printer-friendly .pdf version of this page.
The objective of this bulletin is to describe a method to propagate strawberry plants from runners in deep water culture (DWC). Propagating strawberry plants in deep water culture will reduce grower reliance on soil media and produce strawberry plants that can be directly established into hydroponic systems such as DWC, aeroponics, or nutrient film technique (NFT). In this bulletin we describe a system developed at Cornell University and report on the system performance with two cultivars ‘Albion’ and ‘Cabrillo’ we conclude with challenges and considerations for future optimization.
DWC Runner Production General Tips
- Locate mother plants high off the ground to prevent tangling and prevent contamination from touching the ground.
- Use air stones to aerate the water that runner plants are rooting into.
- Use a reusable plant holder, like GrowGrips, to position runner plants above the water without submerging the crown of the plant, which can cause crown and root rots.
- To control powdery mildew, create a proactive spray regime, making sure to remove dead leaves and debris regularly and spray the top and bottom surface of the leaves once weekly with registered biofungicides (such as: Cease and Milstop SP).
Video Links
- Overview on the operational DWC Runner Propagation Bench
- Planting the mother plants
- Training of runners to grow roots in DWC
- ‘Albion” runner root growth after seven days in the DWC propagation bench
- ‘Cabrillo” runner root growth after four days in the DWC propagation bench and a description of powdery mildew
DWC Runner Propagation Bench
A runner propagation bench was built to continuously propagate new plants of two different day-neutral strawberry varieties, ‘Albion’ and ‘Cabrillo’, for use in deep water culture (DWC) experiments (Figure 1). For each variety, mature “mother” strawberry plants were planted in a mix of 50% perlite and 50% vermiculite in 8” x 16” drip-to-drain rectangular pots at a density of four plants per square foot (6” on center spacing). The pots were arranged in two rows of four with drip irrigation stakes.
The mother plants were irrigated every 12 hours with Jack’s Professional All Purpose LX 21-5-20 Fertilizer at 150 ppm N. The mother plants received supplemental lighting from high pressure sodium (HPS) lamps to maintain 14 hours of daylight. The temperature of the greenhouse was controlled to the elevated set points of 72° F during the day and 65° C during the night to promote vegetative growth and runner production. Flower clusters and dead leaves were removed once every two weeks, so the plants could allocate their energy into runner production. Runners were allowed to grow for two months to generate enough new plants for future experiments.
Deep water culture tubs filled with tap water were set up on either side of the mother plants. One-inch height foam board with two-inch holes drilled three inches on center were placed on top of the tubs (Figure 2). Air stones attached to an air pump were added to the tubs to aerate the water to encourage root growth and prevent rotting. Runners were trained toward the holes and secured with 2” GrowGrips, reusable plastic grips. Special care was taken to position the GrowGrips around the runners so only the very bottom of each runner was below the grip and in contact with the water (Figure 3). This proximity to the water initiates new root formation (Figure 4). If the crown of the runner was positioned too low, the constant wetness could lead to crown rot and poor root formation.




Runner Propagation Results and Discussion
‘Cabrillo’ mother plants were more productive than ‘Albion’, producing about 50% more plants from runners in the same period from December 2019 – February 2020 (Figure 5 and 6). It took two months to produce 100 high quality new plants from runners from 24 ‘Cabrillo’ mother plants at 6” spacing. ‘Cabrillo’ runner plants developed roots faster and developed longer root systems than ‘Albion’ runners (Figure 7). We speculate that a higher infection rate of powdery mildew on ‘Albion’ mother plants and runners may have contributed to ‘Albion’s’ slower growth rate compared to that of ‘Cabrillo’ plants. Once runner plants were positioned in the DWC tubs, it took two weeks for tap roots to develop into 10-20 cm long roots adequate to support plants after transplanting into recirculating DWC systems (Figure 4).
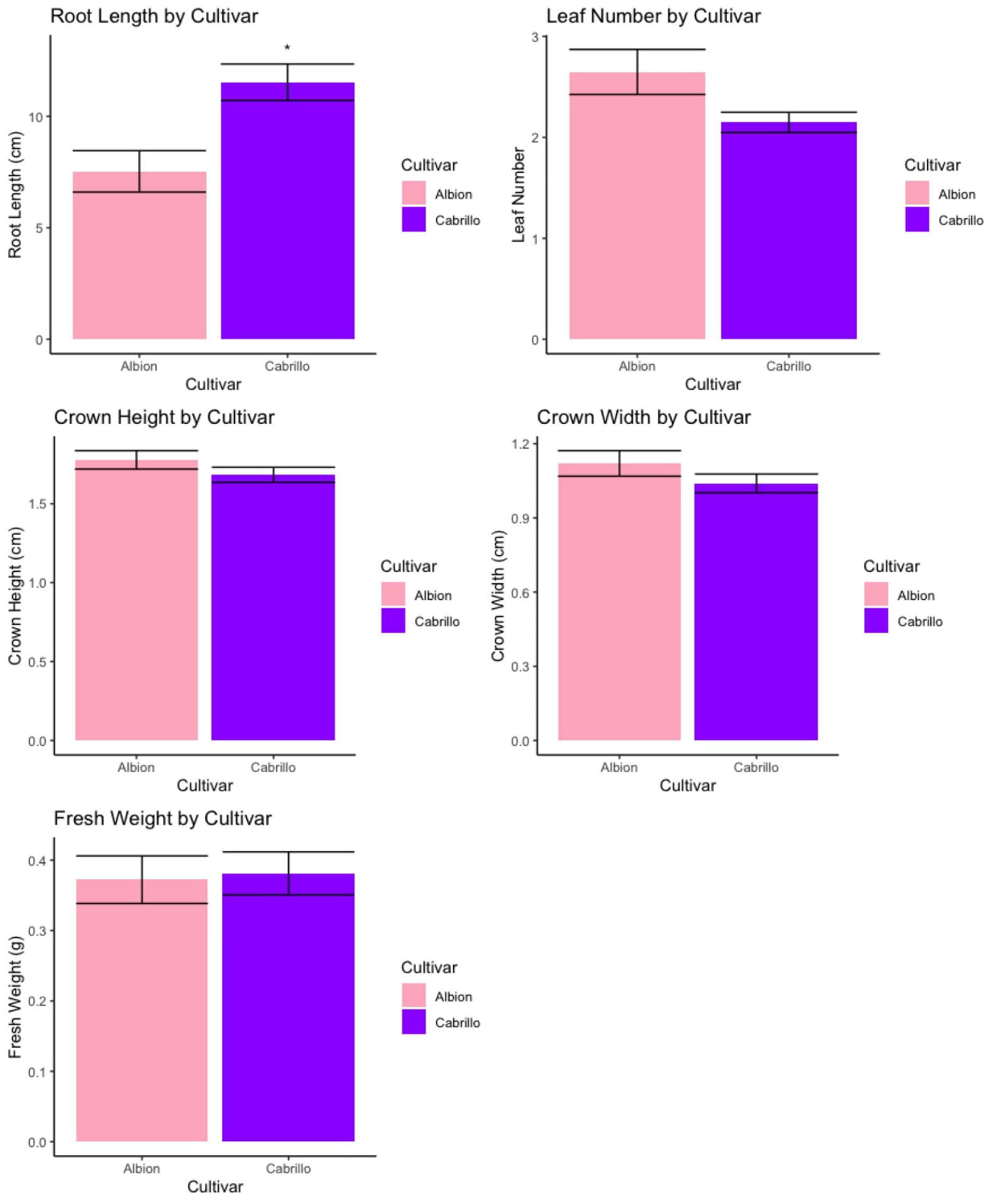
Figure 8 shows the average growth parameters of ‘Cabrillo’ and ‘Albion’ runners (n=55) recorded at the time of transplant into experimental systems. ‘Cabrillo’ runners (mean = 11.5 cm) had significantly longer roots than ‘Albion’ runners (mean = 7.5 cm) after the same period of growth (Figure 8). The effect of cultivar on the other growth parameters such as leaf number, crown height, crown width, and fresh weight of strawberry runners was not significant (Figure 8). In terms of rapid runner production and root growth we recommend using a cultivar like ‘Cabrillo’ for DWC or NFT production. However, we understand some growers prefer ‘Albion’ due to its sweeter flavor.



Challenges and Recommendations
Runner length can become unruly if mother plants are located too close to the ground. Our mother plants were located one meter off the ground and runners had to be bundled so they wouldn’t touch the ground (Figure 9). According to the Kubota Lab’s Controlled Environment Berry Production Information page at Ohio State University (https://u.osu.edu/indoorberry/planting-materials/), Japanese growers produce runners from mother plants located 2 meters off the ground (Figure 10). This elevation would reduce tangling and clutter in the production system.
Powdery mildew (Figure 11) is a common pathogen of greenhouse strawberries and reduces plant vigor. It likely infected plants in the runner propagation bench because of their high density (Figure 9). To control powdery mildew, plant leaves were sprayed once weekly with Cease and Milstop SP. Both are listed by the Organic Material Review Institute (OMRI) as suitable for organic production and both are allowed on edible crops in greenhouses. Cease is a beneficial bacteria (Bacillus subtilis) based biofungicide that colonizes the leaves of plants and secretes antibiotics to prevent infection before a pathogen like powdery mildew can take hold. Milstop SP is potassium bicarbonate and can kill powdery mildew on contact. Note: always check pesticide labels to ensure they are labeled for use in your location and for your specific crop. High densities of tangled strawberry plants prevent the effective application of these products. Leaves that do not get sprayed, especially new growth like runners, cannot be colonized with beneficial bacillus and powdery mildew can infect these susceptible tissues and spread.
Locating mother plants at two meters high off the ground, like that seen in Figure 10, would reduce the need to bundle runners and therefore reduce the density of the new, susceptible plants. We recommend that runners be grown this way for one to two months before lowering down the mother plants and securing the bottoms of the runners in deep water culture foam boards to initiate root growth. Growers should have a proactive spray regime in place, making sure to remove dead leaves and debris regularly.
Algae becomes a concern when the DWC tubs are in continuous operation. Without regular cleaning, algae begins to build up wherever there is light and moisture. Inadequately sized foam boards with too many unoccupied holes or splashing from air stones can accelerate this problem.
We recommend precisely measuring your foam board and cutting it with a new, sharp box-cutter knife to fit inside your deep water tubs without any gaps. Dial in your air stones and air pumps, ensuring they are completely submerged, to prevent excessive splashing. When holes are unoccupied you can cover them with black plastic or another piece of foam board to prevent light from penetrating into the water. Growers should drain and scrub their DWC tubs and flip them over when runner root growth is not being initiated.



Acknowledgements
This work was supported by joint research and extension program under Project No. 1237650 funded by the Cornell University Agricultural Experiment Station (Hatch/Multistate funds) and Cornell Cooperative Extension (Smith Lever funds) received from the National Institutes of Food and Agriculture (NIFA,) U.S. Department of Agriculture (USDA). Support was also provided by a Morley Student Grant and Cornell CALS Charitable Trust Fund. Any opinions, findings, conclusions, or recommendations expressed in this publication are those of the author(s) and do not necessarily reflect the view of USDA. The authors also thank John Osborn, Adam Kulaczkowski, and Dion Santiago Fink for technical assistance.
References and Further Information
Controlled Environment Berry Production Information / Kubota Lab. Ohio State University 2022.
Disclaimer
Mention of trademarks or brand names is for informational purposes only and does not imply its approval to the exclusion of other products that may be suitable.


